This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
HIT Perspectives: ePA 2.0: Taking Electronic Prior Authorization to the Next Level
HIT Perspectives – September 2019
ePA 2.0: Taking Electronic Prior Authorization to the Next Level


By Jocelyn Keegan, Payer Practice Lead,
and Ken Kleinberg, Innovative Technologies Lead
Electronic prior authorization (ePA) is gaining traction and attention after a lull in progress and focus. The need for ePA is easy to understand. ePA is essential in reducing time to therapy, friction and costs by aligning payer and provider goals. The “how” is catching up in a big way. Now new technologies, evolving standards, government regulations and ePA’s role as a critical tool for value-based care have created a perfect storm. The industry has brought ePA to an inflection point, and several leading payers, providers, vendors and standards groups are driving to advance ePA by making automated prior authorization (PA) the norm rather than the exception.
A way to conceptualize this progress is shown in the figure below. There are three phases in the evolution of ePA. The industry is rapidly transitioning from phase 1.0 and heading to phase 2.0.
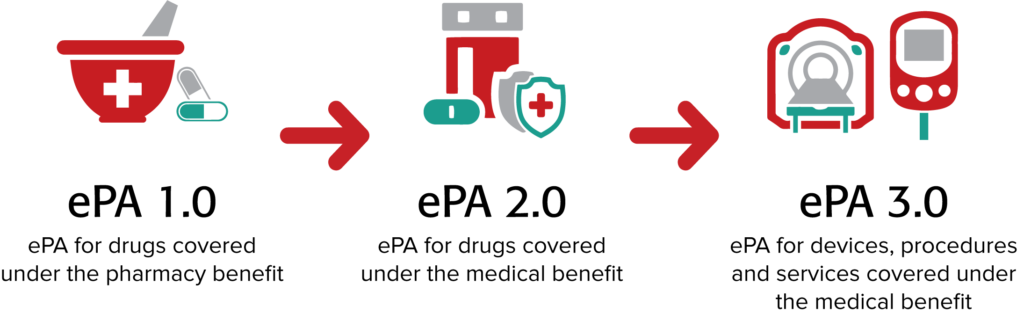
ePA 1.0. This first phase is focused on ePA for medications covered under a patient’s pharmacy benefit. Substantial progress has been made and is ongoing. Take, for example, the latest version of the SCRIPT standard by the National Council for Prescription Drug Programs (NCPDP). NCPDP SCRIPT version 20170701 contains ePA transactions that are more robust than those contained in the current ePA standard (ASC X12 278) and offer the ability for performing ePAs in real time.
Adoption of the NDPDP standard will be reinforced with the newly proposed rule from the Centers for Medicare and Medicaid Services (CMS), which would require use of ePA transactions contained in NCPDP SCRIPT 20170701 for drugs prescribed under Medicare Part D instead of the ASC X12 278. The proposed implementation date is January 1, 2021.
Another CMS rule will support and accelerate use of the real-time pharmacy benefit check (RTPBC). The rule requires adoption of a “Real-Time Benefit Tool” by Medicare Part D plans beginning January 1, 2021. NCPDP has developed a draft standard for this transaction, which was approved to move forward at the August 2019 workgroup meeting.
With the RTPBC, both prescriber and patient can have up-to-date information on the out-of-pocket costs of a drug that is being prescribed as part of the electronic prescribing (ePrescribing) process at the point of care through the electronic health record (EHR). When used with ePA, the two transactions deliver more accurate information about coverage and costs of drugs at the point of prescribing and allow physicians to help their patients begin therapy faster. The newly proposed ePA transactions would enable the prescriber to submit the required information in real time and indicate in the RTPBC whether PA is needed. This will help the physician obtain faster approval and improve speed to therapy.
Improving the quality of the signal for PA through RTPBC will bolster the accuracy and utility of ePA as part of the prescribing process, such as support from industry groups such as the American Medical Association, which continues to support adoption of ePA as a way to reduce physician burden.
Taking ePA to Level 2.0. ePA 2.0 involves automating PAs for drugs covered under a patient’s medical benefit (mPA), such as drugs covered under Medicare Part B. Reducing provider burden is an increasing focus from the Department of Health and Human Services, including notices of proposed rulemaking that would increase the ability for payers to share coverage decisions as members change plans and make continued investments in emerging technologies that can expose coverage rules to providers in their work flow.
Currently, most PAs for those medications are processed manually through antiquated phone, fax and paper processes. There are several drivers propelling ePA to the next level. These are being addressed by various stakeholder initiatives. For example:
- Specialty pharmacy. A large driver for mPA is the rapid growth of expensive specialty medications. Specialty medications are the fastest growing segment of the nation’s drug spend, primarily due to their high costs and use in addressing the large and expanding patient populations with chronic diseases. The government estimates that 60% of Americans have a chronic disease and 40% have two or more chronic conditions.
At the same time, prescribing specialty medications generally is a manual process, leading to provider frustration and reduced speed to therapy. Automating and standardizing specialty pharmacy transactions will be the focus of a new workgroup that has been formed by NCPDP. The goal is to bring greater focus and coordination in how NCPDP standards are used for the electronic exchange of data in specialty pharmacy, including addressing gaps that exist in ePrescribing for specialty medications. This will complement and support ongoing NCPDP efforts to automate various aspects of specialty pharmacy, including the patient enrollment process. In addition, NCPDP and HL7 are working together to use Fast Health Interoperability Resources (FHIR) to extract the necessary clinical data required for enrollment from the native EHR.
- Administrative burdens. Three-quarters of physicians (specialists and primary care) report the burden of PA is high. According to the American Medical Association, doctors and their staff spend the equivalent of nearly two business days navigating PA. Because this administrative burden is so great, about a third of physicians maintain staff members who exclusively deal with PAs. It usually takes days —even weeks — for an insurance company to decide whether it will approve a PA request. Needless to say, this leads to delays in therapy and frustration for both patients and providers. These factors are increasing the demand by provider associations for an mPA solution. Point-of-Care Partners (POCP) is tracking no fewer than a half-dozen separate, industry-led conversations to reduce the need for mPA and its associated provider burden.
- Costs. Significant administrative costs are associated with PA. Research by the Council for Affordable Quality Healthcare reveals that each manual prior authorization for medical care costs $3.50 for plans and $6.61 for providers. Going electronic brings that down to $2.80 per transaction for payers and $0.03 for providers. All in all, the study found that transitioning to electronic medical prior authorization could create $278 million in annual savings for providers and $139 million for health plans.
- Da Vinci. Significant investment from CMS, payers, providers and vendors is under way to accelerate adoption of ePA leveraging application program interface (API)-based standards. HL7’s FHIR is the basic building block for the HL7 Da Vinci Project — a private, multistakeholder initiative — with a number of use cases including PA. Da Vinci’s open business model process enables payers, health systems and other industry participants to identify and enumerate use cases that involve managing and sharing clinical and administrative data among industry partners. Coverage Requirements Discovery, an early use case, leverages a FHIR-based API that enables care delivery organizations and providers to query payers in real time to find relevant guidance prior to care delivery to increase efficient delivery of care and corresponding payment. Building further, Documentation Templates & Rules enables providers to understand coverage requirements for a particular patient at the plan level, and work is under way to map the necessary clinical data required to automate the PA request itself with Prior Authorization Support. All of these use cases are in a single track at HL7’s September Connectathon in Atlanta.
To be sure, this new tranche of work is early, but stakeholders are ramping up to enable their platforms for API access so ePA can move to the next level.
Moving to ePA 3.0. ePA 3.0 will automate PA for devices, procedures and services covered under the medical benefit. Electronic medical prior authorization is in its early phases, but real work is under way with significant interest and attendance at Da Vinci working sessions and Connectathon activities. Many challenges must be addressed to bring ePA to version 3.0. For example:
- The quality of provider data varies by vendor and payer capabilities. Payers will need to streamline and combine how pharmacy and medical claims are processed, as well as increase the accuracy and availability of PA requirements and benefit detail in workflow in real time.
- The increasing complexity of plan design, high-deductible plans, and members in at-risk contracts is increasing pressure on plans to improve available provider tools. Consensus will be needed on what those tools should be, the standards to support the underlying business design and how they will be handled in work flows.
- Refinements are needed in EHRs to support mPA. The ability to seamlessly share or identify in real time existing data locked into EHRs with APIs or data to create necessary attachments must be addressed. EHRs still provide little or no support for PA, except for enabling attachments using existing HIPAA-named standards. Creating a clear path for implementers from FHIR to other ePA standards is critical.
Need more information? POCP is here to help. Drop us a line (jocelyn.keegan@pocp.com and ken.kleinberg@pocp.com). Also, don’t overlook the wealth of information in our new ePA report. This extensive document — with 40+ diagrams and tables and 90+ references — offers health care stakeholders an independent analysis of the market, realistic maturity models and a profile of what vendors and service companies are currently doing pertaining to ePA so they can arm themselves with the information needed to plan strategically and meet their goals. Set up a one-on-one meeting to discuss how this report may help your organization by calling us at 877-312-7627, option 4, or dropping us an email at info@pocp.com.



