This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Biopharma Insights: New Maturity Model Will Help Spur Adoption of Electronic Prior Authorization
Biopharma Insights – June 2017
New Maturity Model Will Help Spur Adoption of Electronic Prior Authorization

By Pooja Babbrah and Jocelyn Keegan, Senior Consultants
An increasing number of medications require preapproval — or prior authorization (PA) — from payers before they can be dispensed. This traditionally has been a cumbersome, time-consuming and frustrating manual administrative process for prescribers, pharmacies and payers. It determines the medications that patients ultimately receive, which are often not the drugs that were initially prescribed. The time lags inherent in today’s paper-phone-fax processes also can create patient safety and quality of care concerns.
That will be changing rapidly due to 1) the overall computerization of healthcare, including nearly ubiquitous use of electronic health records (EHRs), and 2) a new electronic prior authorization (ePA) transaction that can speed advance approval of medications as well as minimize or eliminate hassles and time lags. To help spur adoption among payers and pharmacy benefit managers (PBMs), which are the decision-makers for PA approval, a new ePA maturity model was developed by Point-of-Care Partners (POCP). The model can help these key stakeholders identify their progress with ePA adoption as well as steps they can take to move forward with ePA to optimize their return on investment (ROI).
Drivers for ePA. There are a number of drivers that will increase ePA adoption.
- A standard is in place. Stakeholders from across the industry – led by POCP’s Tony Schueth — developed an electronic prior authorization (ePA) standard using the National Council for Prescription Drug Programs (NCPDP) standard for electronic prescriptions (SCRIPT). The ongoing goal is to incorporate the standard into the electronic health record (EHR) work flows of physicians, pharmacies and payers. A draft standard was piloted in 2011, and the standard became a reality a couple of years later. As the standard has matured over the years, EHRs and payers have become increasingly able to handle ePA transactions.
- The business case is clear. There are significant administrative costs associated with manual PA processing, which can be mitigated by computerized processing. According to a recent article in Health Affairs, physicians spend the better part of $37 billion annually ($83,000 per doctor) thrashing out PA and formulary issues with payers. According to another estimate, doctors spend 868.4 million hours on PA each year — not counting time devoted by other staff members. ePA reduces the time spent on each PA by all participants. A survey by the American Medical Association indicated that most physicians experience a delay in excess of a week for their PA request to be processed. PA also is a work-flow drag on pharmacies. In contrast, prospective ePA — which allows advance approval of an ePA request — often can be processed within minutes when payers are equipped to electronically accept and process PA requests, as well as return real-time responses using the NCPDP standard.
- Patient safety and quality of care concerns. The difficulties inherent in trying to obtain a PA significantly affect patient care and safety. Nearly 40% of PA requests (roughly 75 million) annually are abandoned due to complex procedures and policies and the hassle factor. Moreover, nearly 70% of patients encountering paper-based PA requests do not receive the medication originally prescribed.
- State legislative mandates. States also are beginning to recognize the benefits of ePA based on the NCPDP standard. Eleven have enacted laws mandating payer support for prescriber-initiated NCPDP-formatted PA transactions. In a newly enacted law, Indiana is going a step further with requirements for ePA transactions initiated by pharmacists. Beginning on January 1, 2018, health plans in the state must accept and respond to NCPDP-formatted ePA requests from a prescriber or dispensing pharmacist. Indiana’s statute does NOT mandate pharmacist use of this approach or say other methods are possible nor prohibited. Even so, this is another step toward promoting widespread adoption of ePA. States watch each other. Once the ice has been broken with respect to a particular regulatory approach, other states generally follow suit. In addition, an NCPDP workgroup is moving forward with development of a use case for ePA, based on the NCPDP standard, initiated by clinical pharmacists in the postacute care setting. Such activities should also hasten more widespread and complete adoption of prospective ePA.
- Rise of specialty medications. Specialty medications are the fastest growing segment of the nation’s drug spend, primarily due to their high costs and use in addressing the large and expanding patient populations with chronic diseases. Most specialty medications require PA. Automating the PA process will have increasing appeal to pharmacies, prescribers and payers alike. They will want to keep ahead of the curve by incorporating ePA for specialty medications into their workflows as well as improve the quality and safety of patient care.
Barriers to ePA adoption. It is clear that the time is right for stakeholders to invest in prior authorization automation. However, not all ePA is equal. Today, anything “electronic” related to PA may be inaccurately considered ePA. Despite the availability and adoption of the NCPDP ePA standard, the current process remains largely reactive, with providers and pharmacies chasing failed electronic prescriptions because they need a PA.
There are a number of reasons why PA remains a challenge for payers to move forward with prospective ePA. Each payer has evolved its use of ePA through a myriad of business and technology drivers, lines of business and customer-specific constraints. There are also varying state and local regulatory requirements. States continue to enact requirements for ePA for pharmacy and medical benefits, but the rulings vary by state and often do not mandate use of ePA based on the NCPDP standard. In addition, payers struggle with integrating ePA into internal systems — whether they are installed as software or home-grown utilization management systems. Potential inaccuracies of some data concerning PA in electronic prescribing work flows — such as the need for PA for a particular patient —are often confusing or inconclusive for prescribers. Finally, users continue to grapple with EHR design challenges, which both delay and suppress physicians’ adoption of prescriber-driven ePA.
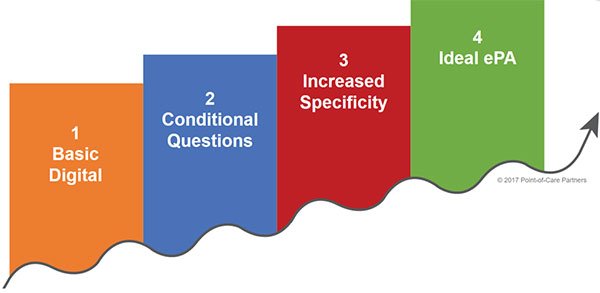
The ePA maturity model. Based on interviews with PBMs, POCP developed an ePA maturity model. With it, payers and PBMs can understand where they are across four levels of maturity (as shown below) and learn more about proven best practices to improve the ROI on their ePA investment. One payer saw a 28% improvement in automation by reengineering supporting processes.
The model is customizable, incorporates NCPDP standards and is designed so users can identify and address gaps that are preventing PA from moving toward a totally electronic process.
Using the ePA maturity model allows payers and PBMs to:
- Create a framework to categorize improvement opportunities. This provides a tool to visualize opportunities across business or client-selected categories. It also allows PBMs to identify patterns for programmatic investment and opportunities. This, in turn, allows them to acknowledge and understand areas of the business so as to avoid or apply only proven best practices. The framework can help identify lines of business that are ripe for experimentation.
- Honestly assess adoption of ePA. Implementation varies among EHRs and providers. Even though the ultimate goal is full automation of the ePA process, outcomes may be improved at every step of the PA process. In fact, any incremental improvement to improve true ePA adoption results in real savings and operational efficiency.
- Determine where organizational adoption lies on the ePA maturity curve. The maturity curve has four inflection points ranging from basic digital ePA to use of conditional questions, to use of more specific questions, and finally to ideal and fully automated ePA using the NCPDP standard. This will allow payers to see at a glance where their organization lies in terms of real ePA adoption and where forward progress can be made.
- Develop and deliver organization-specific recommendations using proven best practices. Using data gleaned from steps 1-3, the organization can assess its status and what must be done to move forward. The goal is to move all process and question sets as far up the maturity curve as possible. For example, the ePA maturity model can help PBMs identify potential lines of business that are ready for process improvement, discuss top strategic goals for PA and explore partnerships or pilots.
Importance of the ePA maturity model. Pharmaceutical companies will want to pay attention to ePA and its growing maturity in the market. For example,
Any delay in therapy adversely affects adherence, patient satisfaction and ultimately patient outcomes.
- 70% of prescriptions rejected at the pharmacy require PA; 40% of those prescriptions are eventually abandoned due to the antiquated and complex, paper-based PA process.
- The PA process impacts more than 185 million prescriptions each year with nearly 75 million abandoned prescriptions.
ePA adoption will significantly affect which drugs are approved and dispensed.
- Electronic prior authorization will empower physicians and patients to discuss appropriate treatment at the point of care, allowing the physician to select the medication best suited for treatment while at the same time considering the patient’s cost factors.
- Well-formed, specific, and codified question sets from payers are a critical success factor for ePA adoption. Payers that are further along on the adoption curve will have honed and standardized their PA question sets, which in turn will speed time-to-fill in pharmacies and patient pick-ups of medications.
- Mature organizations will be prepared to handle and create value from the richer patient data sets and coded data criteria from a patient’s record to make a more accurate ePA determination. This is essential in the growing move toward value-based care.
Finally, ePA is coming sooner rather than later. The vast majority of PBMs now support ePA for retail drugs. There is movement to begin the automation of specialty pharmacy, where the use of the ePA transaction will be key to reducing costs and turnaround.
Moving forward. To be sure, there is much more detail surrounding each section of POCP’s ePA maturity model. POCP can provide a consistent, outside-in assessment on how to move an organization and its partners forward with investments at the points of highest potential return. Let us know how we can put the ePA maturity model to work for you.
Need to keep up with state laws and regulations concerning ePA and other issues affecting prescribers? Let POCP’s Regulatory Resource Center (RRC) do the work for you. The RRC helps clients stay up to date with frequent changes in state and national regulatory requirements through such service offerings as the ePrescribing State Law Review and the ePA State Navigator. The RRC team members can help you quickly uncover answers to your most pressing regulatory questions. All clients in our premium portfolios receive complimentary access to our regulatory team members who are fully committed to providing the utmost quality in tracking and defining regulatory issues. Find out more from the RRC’s director, Connie Sinclair (connie.sinclair@pocp.com).




