This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
ePrescribing State Law On-Demand
- August 22, 2017
- By Connie Sinclair
- 0 Comment
Quick Reference Tables of U.S. Laws and Regulations Governing ePrescribing
The State Law On-Demand research brief details enacted ePrescribing regulations for all 50 states and Washington, DC. Updated within the most recent 3 months, this one-time snapshot provides easy-to-reference data that simplifies regulatory tracking and helps development teams to be proactive in planning for system updates.
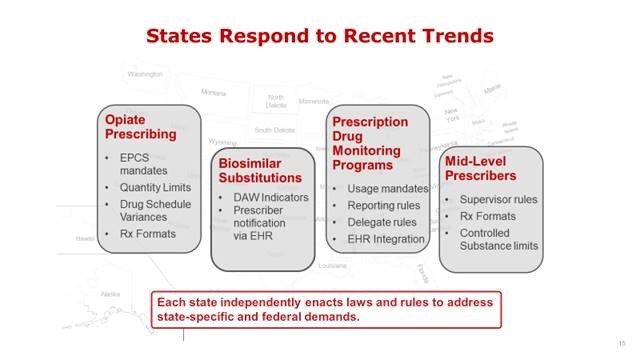
In recent years, states began to enact new types of regulation to expand ePrescribing use, address opioid abuse and to support biosimilars. This year has been an explosive year for new legislative mandates, many of which directly impact how medications are prescribed through electronic health records (EHRs). The trend is rapidly accelerating, particularly as states attempt to reign in the opioid epidemic.
In 2017, 265 new ePrescribing regulations were issued across states, nearly triple the number issued in 2016.
Monitoring state ePrescribing legislation has always been important for vendor compliance for such things as prescription print and fax formats and rules regarding the ePrescribing transaction. All these regulations vary considerably by state and require constant monitoring to ensure products remain compliant and avoid situations that could place prescribers at risk for non-compliance.
What’s the benefit?
- Knowing in advance about regulatory changes that affect your product and your clients
- Additional validation that your product complies with local and federal regulations
- Significant time and cost savings associated with last-minute software updates to address unexpected regulatory changes
- Cost-effective and efficient tool to maintain client satisfaction
What’s Included?
A series of tables that provide a current snapshot of regulations for:
- Formats for printed prescriptions
- Requirements and mandates for delivery of faxed and electronic prescriptions
- Do not substitute/DAW designation requirements
- Mid-Level Practitioner prescribing limits (Physician Assistants, Advanced Nurse Practitioners)
- Prescription Drug Monitoring Program (PDMP) requirements for prescribers
- Controlled substances prescribing limits
- Biosimilar substitution designation and reporting requirements
- State-specific controlled substance schedule variances
- Contact details for state agencies (Board of Pharmacy, PDMP Programs)
Law On-Demand Examples
Add the Law On-Demand to Cart:
Click here for a comparison of Point-of-Care Partners’ ePrescribing regulatory solutions or visit these pages:
Regulary Resource Center
ePrescribing State Law Review
ePA State Navigator
LTPAC State Navigator
Connie Sinclair
State Law On-Demand
Is our latest research brief for state ePrescribing regulatory profiles for all 50 states and Washington, DC.
Do you need a more comprehensive, ongoing subscription solution that includes one-on-one consulting support? The ePrescribing State Law Review combines access to Point-of-Care Partners regulatory experts with timely analyses of evolving laws and regulations governing electronic prescriptions.
Click here for a comparison of these products.