HIT Perspectives: The Impact of Cost on EPCS Adoption
HIT Perspectives – April 2018
The Impact of Cost on EPCS Adoption
 By Michael Burger, Senior Consultant
By Michael Burger, Senior Consultant
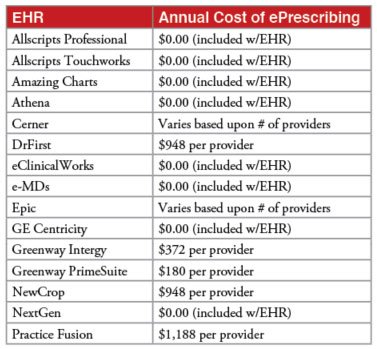
Table 1. Costs for basic ePrescribing in the most widely used EHRs. Source: Point-of-Care Partners.
Correction: The “Costs associated with EPCS” table (Table 2) originally misstated the costs for DrFirst. The actual cost is $90 one-time setup fee, $75 annual ongoing cost, token included in the setup fee.
Healthcare is rapidly going digital. However, electronic prescribing for controlled substances (EPCS) is lagging way behind this trend. Surescripts data show that only 14 percent of prescribers are utilizing EPCS, as compared with 64 percent who prescribe “basic” prescriptions electronically (ePrescribing), or ePrescribing for noncontrolled substances.
These statistics show that EPCS has a long way to go to reach the tipping point. Why is EPCS adoption so low, even though it has been legal since June 1, 2010? The most frequently cited reason is cost. Yet there could be other factors affecting uptake.
Here are three things to know concerning the costs of EPCS and other factors that may affect adoption. The analysis is based in part on research by Point-of-Care Partners.
- Costs associated with basic ePrescribing. Both basic ePrescribing and EPCS typically are features within electronic health record (EHR) software. Virtually all EHRs include basic ePrescribing. EPCS is often an add-on that prescribers can request. For many EHRs, basic ePrescribing is included in the price of the software. As shown in Table 1 (below), others charge annual fees ranging from $180 per provider to as much as $1,200 per provider, with the majority falling somewhere in the middle.
- Costs associated with EPCS. EPCS systems must meet stringent Drug Enforcement Administration (DEA) requirements for credentialing, software certification and dual factor authentication. To cover those costs many EHR vendors have imposed fees, which vary widely by product and vendor. Often, basic ePrescribing is included in the EHR without a specific itemized fee. EPCS sometimes follows the same model, although a surcharge is common. In some cases, the authentication token needed for EPCS is included in the surcharge; in others, it is priced separately. The following is a summary of costs associated with EPCS in the most widely used EHRs, estimated to represent about two-thirds of ambulatory EHR market share.
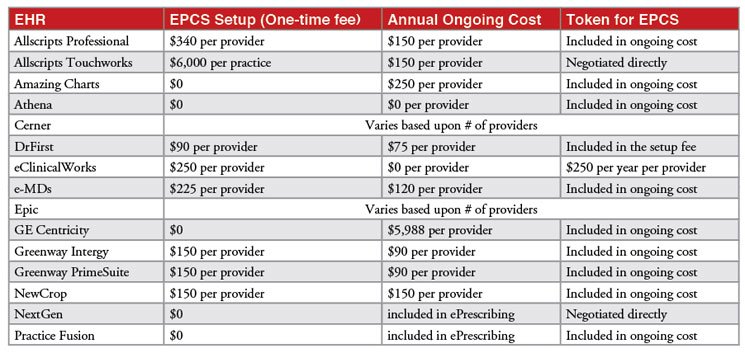
Table 2. Costs associated with EPCS in the most widely used EHRs. Source: Point-of-Care Partners.
3. Going beyond costs. Costs are a major decision factor in adopting health IT. However, there are potentially offsetting factors to be considered, such as the following:
- Return on investment (ROI). Positive early ROI analyses are emerging. An example is ROI results presented at the March 2018 meeting of the Healthcare and Information Management Systems Society by Geisinger Health, which is a large integrated delivery system (IDS) in Pennsylvania. Geisinger demonstrated calculated savings of $1 million in the first year as a result of implementation of EPCS across 126 clinics and 1,661 physicians. These savings were achieved through reductions in call center and diversion control human resources, as well as prescribing efficiency for physicians and nurses. Using EPCS, prescribers reduced the amount of time spent per controlled substance prescription from 3-5 minutes to 30 seconds. In addition to improving provider satisfaction, EPCS enhanced patient safety and care quality through better pain management and a significant reduction in opioid prescriptions. These are important measures — not only in and of themselves, but also as factors upon which providers will be measured and reimbursed as healthcare transitions to value-based care.
- Mandates. In an effort to fight the opioid epidemic, several states have followed New York’s lead and are mandating — or are soon to mandate — EPCS. According to data from POCP’s Regulatory Resource Center, four are mandating EPCS and another five have passed legislation requiring electronic prescribing for opioids. Other states are considering jumping on the bandwagon. Legislation is pending in Congress to mandate EPCS for Medicare Part D, in part as a way to address the opioid crisis. Regardless of cost concerns, mandates will drive adoption of EPCS.
Conclusion. For a provider who writes few controlled substance prescriptions, the costs and fees for EPCS, as outlined above, may not be viewed as cost effective. However, that may be different for the typical provider, for whom controlled substance prescriptions account for 15% to 20% of prescription volume. For such providers, the cost of adding EPCS is not exorbitant on a per-prescription basis.
Thinking in terms of addressing a national public health crisis, the cost of EPCS is small in comparison to those of treating opioid addiction, unnecessary emergency department visits and hospitalizations for overdoses, and lost productivity due to death and disability. These are estimated to cost the US economy more than $500 billion annually.
Ultimately, cost may not be the only factor affecting EPCS adoption. As healthcare transitions to value-based reimbursement, the costs of EPCS must be viewed in a broader context. This includes costs versus benefits for patient care and quality, reimbursement based on metrics related to EPCS and the requirements of value-based care organizations and IDSs for implementing EPCS using specific EHRs.
Finally, the costs of EPCS adoption may be a moot point if EPCS is mandated at the state and federal levels. However, increased uptake due to mandates may drive down implementation costs, but this will be on a longer-term horizon.
Need to keep up with state EPCS mandates? Our Regulatory Resource Center team members can help you quickly uncover answers to your most pressing regulatory questions. All clients in our premium portfolios receive complimentary access to our regulatory team members who are fully committed to providing the utmost quality in tracking and defining regulatory issues. Drop an email to Keith Fisher (keith.fisher@pocp.com) and we can show you how our expertise and data can meet your needs.


